1. Development of battery industry
Battery manufacturing industry is not only a traditional industry, but also an important part of new energy industry in China. It is closely related to new energy vehicles, renewable energy, modern electronic information, new materials, equipment manufacturing and other strategic emerging industries. Battery manufacturing industry is also the most important basic industry in China's national economic construction, which is related to the national economy and people's livelihood and the foundation of building a well-off society; Battery products have a wide range of applications and very important role in adapting to the development of national economy under the new situation of our country, ensuring the strategic needs of national defense, and meeting the diversified needs of public work and living consumption. The battery includes physical battery and chemical battery. Physical battery is a device that directly converts solar energy, heat energy or nuclear energy into direct current energy by using physical effect, such as solar cell, thermoelectric generator, nuclear cell, etc.; chemical battery is a device that directly converts chemical energy into direct current energy, such as lead-acid battery, lithium-ion battery, zinc manganese battery, etc. In the battery, chemical battery is the most important one. Chemical batteries can be divided into primary batteries and secondary batteries according to whether they can be recycled. Among them, the primary battery is the battery that the active substance can only be used once, also known as primary battery, such as zinc manganese battery, alkaline manganese battery, etc.; the secondary battery can be recharged and recycled, also known as battery. The battery uses the chemical reaction of the active substance in the battery to output current in the state of discharge, and the reverse chemical reaction in the state of charge to store electric energy.
According to the different electrode materials and working principles, batteries are mainly divided into four categories: lead-acid batteries, lithium-ion batteries, Ni MH batteries and Ni Cd batteries. Among them, lead-acid battery has the advantages of high cost performance, large capacity, high power, long life, safety and reliability, which is the largest production and most widely used battery in the world; lithium ion battery also occupies a certain market share with the advantage of high energy density.
2. Overview of lead acid battery
Composition and working principle of lead acid battery
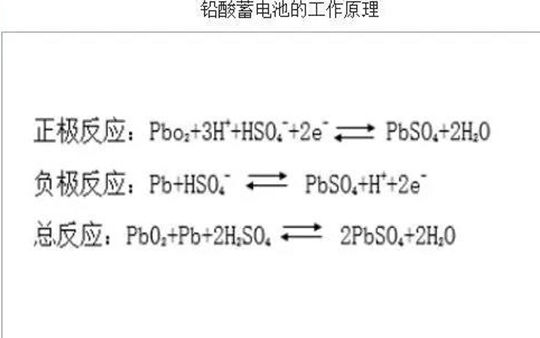
Lead acid battery is composed of positive plate, negative plate, separator, electrolyte, plastic tank, etc. The positive active material of lead-acid battery is lead dioxide (PbO2), and the negative active material is lead (PB). The electrolyte is dilute sulfuric acid. The positive and negative electrodes are separated by a separator. The ions in the electrolyte can pass through the micropores in the separator, and the electrons on the electrode cannot pass through the separator. After the lead-acid battery is discharged, the active material PbO2 of the positive plate is transformed into lead sulfate (PbSO4) which is attached to the positive plate, and the negative active material Pb is also transformed into lead sulfate (PbSO4) which is attached to the negative plate. The sulfuric acid in the electrolyte diffuses into the plate, and the concentration of the electrolyte decreases. When the lead-acid battery is charged, the opposite reaction occurs. Through charging and discharging reaction, lead-acid batteries can be used repeatedly until the storage capacity can not meet the requirements of electrical appliances, and the service life is terminated.

The standard voltage of lead-acid battery is 2V. In order to meet the needs of high voltage of electrical appliances, batteries are often combined in series into 6V, 12V and other batteries; in order to meet the needs of high capacity of electrical appliances, it is often realized by increasing the area of plates or welding the same plates in parallel into a group of electrodes. When a fully charged battery is discharged to a specified voltage according to certain discharge conditions, the amount of electricity released is called the capacity of the battery, and the unit is generally ampere hour (abbreviated as ampere hour, denoted by "ah"). The ability of a battery to release electricity is called energy, which is the capacity of the battery multiplied by the average discharge voltage, usually expressed in volt ampere hour (VAH) or kilovolt ampere hour (KVAh).